Histone bivalency in neuronal development
During development, neurons undergo a series of functional and morphological changes to become mature neurons integrated into brain circuitry. The developmental progression of neuronal precursors is regulated by changes in gene expression, which, in turn, are controlled by the chromatin landscape. We are interested in understanding how chromatin mechanisms regulate transcriptional programs during neuronal development.
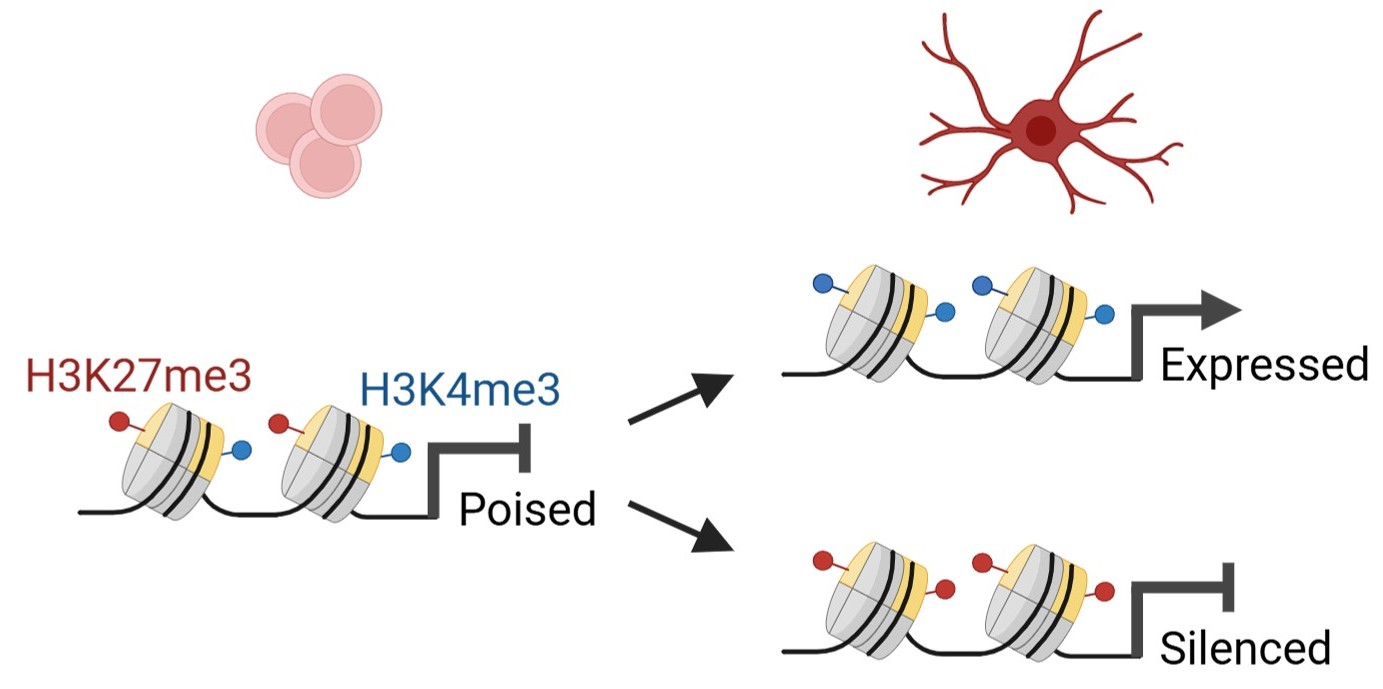
Our work has revealed the importance of histone bivalency in regulating the appropriate timing of gene expression programs during neuronal maturation. Histone bivalency refers to the colocalisation of activating and repressive modifications on chromatin, and it is thought to maintain gene expression in a ‘paused’ state. Our studies on the cerebellum showed that if bivalency is perturbed, developing neurons skip glial-guided migration, an essential step in cerebellar cortex formation, and instead undergo premature maturation. We are now identifying novel bivalency regulators and exploring how bivalency is regulated in mouse and human neurons.
Chromatin dynamics in developing and ageing neurons
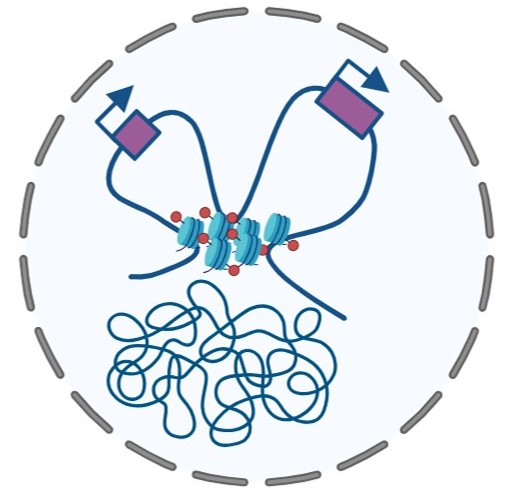
Neuronal maturation is accompanied by dynamic changes in chromatin 3D architecture and epigenetic modifications. These changes establish the transcriptional programs that control neuronal function. We are interested in how global and local changes in histone modifications such as H3K27me3 control chromatin interactions and gene expression. To address these questions, we use a variety of methods such as biochemistry, imaging, genome-wide sequencing, and bioinformatics in primary neurons and animal models. With these studies, we aim to define how chromatin mechanisms guide neuronal maturation and control neuronal identity and function during adulthood and ageing.